- Product Details
Keywords
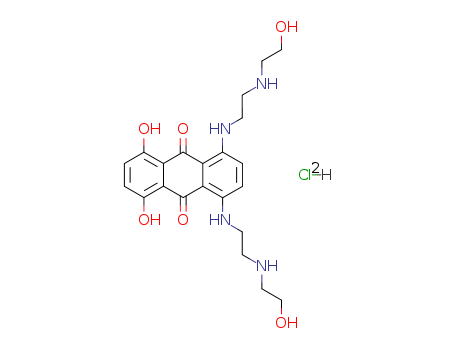
- Mitoxantrone hydrochloride
- Mitoxantrone HCL
- 9,10-Anthracenedione,1,4-dihydroxy-5,8-bis[[2-[(2-hydroxyethyl)amino]ethyl]amino]-, dihydrochloride(
Quick Details
- ProName: Mitoxantrone hydrochloride
- CasNo: 70476-82-3
- Molecular Formula: C22H28N4O6. 2HCl
- Appearance: Dark blue,electrostatic powder
- Application: oncology,Anti-cancer
- DeliveryTime: Prompt
- PackAge: 500g/Alu Tin
- Port: Shanghai, Wuhan, Hangzhou
- ProductionCapacity: 3 Kilogram/Month
- Purity: 99%
- Storage: Stored with sealed
- Transportation: By air
- LimitNum: 20 Gram
Superiority
ISO9001
|
Certification Name |
Certified By |
Certificate No. |
Product Name |
Available Date---Expired Date |
|
ISO 9001:2008 |
Moody Internaional Certification Ltd. |
121001006 |
Development and Production of API |
2010/04/01---2013-03/31 |
ISO14000
|
Certification Name |
Certified By |
Certificate No. |
Product Name |
Available Date---Expired Date |
|
ISO 14001:2004 |
Moody Internaional Certification Ltd. |
111001038 |
Development and Production of API |
2010/06/17---2013-06/16 |
OHSAS18000
|
Certification Name |
Certified By |
Certificate No. |
Product Name |
Available Date----Expired Date |
|
BS-OHSAS 18001:2007
|
Moody Internaional Certification Ltd. |
7648 |
Development and Production of API |
2010/06/16---2013-06/15 |
Details
【Company Profile】
Company Name: Hubei Haosun Pharmaceutical Co., Ltd.
Year Established: 2003
Main Products: Active pharmaceutical ingredients, completed product, cosmetics health food, etc.
【Specification】
Purity: 99%
Appearance: Dark blue,electrostatic powder
Standard:USP35;
Certificate:Mexico GMP (Approved by COFEPRIS);
Manufacturing site approved by FDA, SFDA, MHRA, COFEPRIS
【Application】
It is used in the treatment of certain types of cancer, mostly metastatic breast cancer, acute myeloid leukemia, non-Hodgkin's lymphoma. It was also shown to improve the survival of children suffering from first relapse of acute lymphoblastic leukaemia.
ISO9001

ISO14000

OHSAS18000