- Product Details
Keywords
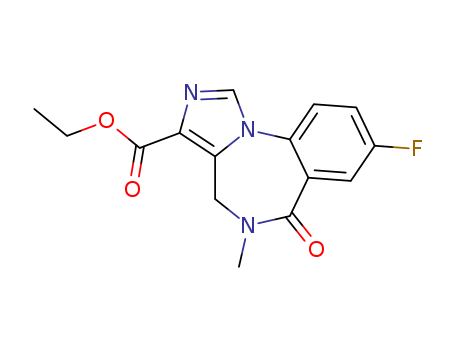
- Flumazenil
- Ethyl 8-fluoro-5,6-dihydro-5-methyl-6-oxo-4H-imidazo(1,5-a)(1,4)benzodiazepine-3-carboxylate
- 4H-Imidazo(1,5-a)(1,4)benzodiazepine-3-carboxylic acid, 8-fluoro-5,6-dihydro-5-methyl-6-oxo-, ethyl
Quick Details
- ProName: Flumazenil
- CasNo: 78755-81-4
- Molecular Formula: C15H14FN3O3
- Appearance: White crystalline powder
- Application: Selective antagonists
- DeliveryTime: Prompt
- PackAge: 100g/Alu Tin
- Port: Shanghai, Wuhan
- ProductionCapacity: 1 Kilogram/Month
- Purity: 99%
- Storage: Preserve in tight container
- Transportation: By air
- LimitNum: 10 Gram
Superiority
ISO9001
|
Certification Name |
Certified By |
Certificate No. |
Product Name |
Available Date---Expired Date |
|
ISO 9001:2008 |
Moody Internaional Certification Ltd. |
121001006 |
Development and Production of API |
2010/04/01---2013-03/31 |
ISO14000
|
Certification Name |
Certified By |
Certificate No. |
Product Name |
Available Date---Expired Date |
|
ISO 14001:2004 |
Moody Internaional Certification Ltd. |
111001038 |
Development and Production of API |
2010/06/17---2013-06/16 |
OHSAS18000
|
Certification Name |
Certified By |
Certificate No. |
Product Name |
Available Date----Expired Date |
|
BS-OHSAS 18001:2007
|
Moody Internaional Certification Ltd. |
7648 |
Development and Production of API |
2010/06/16---2013-06/15 |
Details
【Company Profile】
Company Name: Hubei Haosun Pharmaceutical Co., Ltd.
Year Established: 2003
Main Products: Active pharmaceutical ingredients, completed product, cosmetics health food, etc.
【Specification】
Standard: EP/USP;
Manufacturing site approved by FDA, SFDA, MHRA, COFEPRIS
Certificate:
DMF No.17971 (Approved by USFDA)
CEP: R0-CEP2008-121-Rev01
Approved by USFDA & MHRA, got UK GMP
【Application】
It is used as an antidote in the treatment of benzodiazepine overdoses It reverses the effects of benzodiazepines by competitive inhibition at the benzodiazepine binding site on the GABAA receptor. There are many complications that must be taken into consideration when used in theacute care setting. It has also been used in hepatic encephalopathy, though results have been mixed.
ISO9001

ISO14000

OHSAS18000