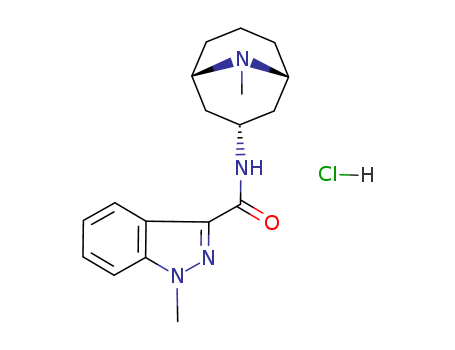
Granisetron hydrochloride CAS NO.107007-99-8
- Min.Order: 50 Gram
- Payment Terms: L/C,T/T,
- Product Details
Keywords
- Granisetron hydrochloride
- 1H-Indazole-3-carboxamide,1-methyl-N-(9-methyl-9-azabicyclo[3.3.1]non-3-yl)-, monohydrochloride, end
- BRL 43694A
Quick Details
- ProName: Granisetron hydrochloride
- CasNo: 107007-99-8
- Molecular Formula: C18H25ClN4O
- Appearance: White cystalline powder
- Application: Antiemetic
- DeliveryTime: 10~15days; Prompt
- PackAge: 500g/Tin
- Port: Shanghai, wuhan, hangzhou
- ProductionCapacity: 4 Kilogram/Month
- Purity: 99%
- Storage: Stored with sealed
- Transportation: By air, express
- LimitNum: 50 Gram
Superiority
ISO9001
|
Certification Name |
Certified By |
Certificate No. |
Product Name |
Available Date----Expired Date |
|
ISO 9001:2008 |
Moody Internaional Certification Ltd. |
121001006 |
Development and Production of API |
2010/04/01----2013-03/31 |
ISO14000
|
Certification Name |
Certified By |
Certificate No. |
Product Name |
Available Date----Expired Date |
|
ISO 14001:2004 |
Moody Internaional Certification Ltd. |
111001038 |
Development and Production of API |
2010/06/17----2013-06/16 |
OHSAS18000
|
Certification Name |
Certified By |
Certificate No. |
Product Name |
Available Date----Expired Date |
|
BS-OHSAS 18001:2007
|
Moody Internaional Certification Ltd. |
7648 |
Development and Production of API |
2010/06/16----2013-06/15 |
Details
【Company Profile】
Company Name: Hubei Haosun Pharmaceutical Co., Ltd.
Year Established: 2003
Main Products: Active pharmaceutical ingredients, completed product, cosmetics health food, etc.
【Specification】
Standard: EP7.5 / USP35
Manufacturing site approved by FDA, SFDA, MHRA, COFEPRIS
Certificate:
DMF No.19365 (FDA approved);
CEP:R0-CEP2009-341-Rev00 (EDQM approved);
MHRA approved, got UK GMP;
【Application】
Granisetron Hydrochloride is a highly s elective 5-HT 3 receptors inhibitor. It is favourable effective fthe prevention the treatment of nausea vomiting caused by actinotheraphy, chemotherapy surgical.
ISO9001

ISO14000

OHSAS18000